Unraveling Cosmic Chemistry: New Insights into Formaldehyde Formation
Imagine peering into the cold, dark corners of space, where stars are just beginning to flicker into existence. It is in these cosmic nurseries where key chemical species formaldehyde (H₂CO) and methanol (CH₃OH) are forged. These precursor molecules are vital ingredients for brewing the more complex organic molecules that are essential to understanding how life might have originated on Earth or, perhaps, elsewhere in the universe. Now, thanks to revisions in the chemical model ‘MONACO’, a team of scientists, led by Anna Punanova, found that a specific gas-phase reaction is vital for matching the observational values of H₂CO in molecular clouds.
In cold molecular cloud environments, H₂CO forms both through chemical reactions on the surfaces of icy dust grains, as well as reactions occurring entirely in the gas-phase. CH₃OH forms entirely on dust grains. The multiple formation pathways of H₂CO make modeling the chemistry more complicated, since each possible pathway contributes different amounts of H₂CO. Their formation, however, is closely linked to carbon monoxide (CO). As CO “freezes out”, when its gas-phase molecules stick onto the dust grains in cold clouds, the abundances of H₂CO and CH₃OH decrease. This phenomenon has been observed in the Taurus Molecular Cloud using the IRAM 30m telescope in Spain.
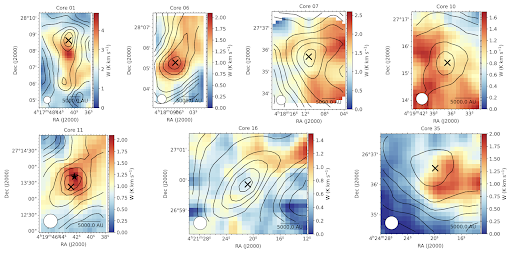
Figure 1: From Punanova et al., 2025 a series of maps showing the formaldehyde integrated intensity overlaid with contours showing the visual extinction.
Initially, the MONACO model that accounted for these CO formation routes overpredicted H₂CO abundances by more than an order of magnitude when compared to observed values coming from the IRAM telescope. Punanova and her collaborators investigated various parameters thought to influence H₂CO formation and destructions, such as cosmic ray ionization rate, elemental abundances, metallicity, and gas temperature—but found no significant difference. The breakthrough came only by including an additional channel to account for the dominant gas-phase reaction between the methyl radical, CH₃, and the oxygen atom, O, which then allowed the modeled H₂CO abundances to better match observations.
While these new changes to the chemical models have brought H₂CO abundances closer to the observed values, some variations by factors of a few still remain, suggesting that scientists might still be missing a piece of the puzzle. These remaining overestimations are thought to be a consequence of either overestimating the formation rate or underestimating the destruction rate of H₂CO. Still, the updates mark an important step forward in modeling the chemistry in cold environments. Continued investigation into environmental conditions and their application to these models will further enhance our understanding of the underlying chemistry that traces our cosmic origins.
This article made use of the following publication:
Punanova. A.F. et al., 2025, Monthly Notices of the Royal Astronomical Society, 537, 4, 3686-3700Original Contributor

Lucille Steffes
University of Arizona
Editors
Samantha Scibelli
Jansky Fellow at the NRAO
Annika Geiger
The Astrochemistry Report