Predicting the Missing Sulfur Reservoir with Synthetic Observations and Laboratory Experiments
Picture yourself baking a cake. You follow the recipe exactly, excited to cook the perfect dessert. As you add all of the ingredients, you realize something crucial: You don’t have flour! Your whole plan is ruined. How can you bake a cake if you’re missing one crucial element in the recipe? This exact problem has troubled astrochemists and astrobiologists for more than 40 years. Instead of flour – the missing ingredient is sulfur. The current thought is that sulfur is “hidden” in other molecules in space rather than existing in its elemental form. Researchers at the Centro de Astrobiología in Madrid, Spain, led by Asunción Fuente, believe they have identified in which form sulfur is concealed in the galaxy. Just as flour is vital for baking, sulfur plays a key role in building life’s ingredients -- and now, we might finally know where it’s been hiding.
What is the Missing Ingredient of Life In Space?
Life is mostly made up of the combination of six atoms : carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur. In space, we have a global idea about how these elements are distributed. For example, the amounts of carbon, oxygen, and nitrogen in the interstellar gas are known in our galaxy. In contrast, sulfur is the tenth most abundant element in the universe and a building block of biological systems, yet it is the only element in which the gas-phase abundance - the concentration of molecules or atoms in a gaseous state - is uncertain by several orders of magnitude.
The gas-phase abundance of each atom is derived from observations of the Sun's photosphere - the visible surface of the Sun - and from analysis of meteorites and the Earth’s crust. Observations of other stars have shown that they have almost the same chemical composition as the Sun, so from there, astrochemists started using the Sun’s chemical abundance as a proper estimate of what we should expect in our galaxy. However, when astrochemists compare the gas-phase sulfur abundance to other parts of the interstellar medium such as molecular clouds, dense cores, protostars and even protoplanetary disks, nothing comes near this abundance. The abundances of gas and ices in space is far lower than what is observed in the Sun's photosphere. Furthermore, from a molecular cloud to a protostellar disk, where a star is forming, sulfur is nowhere to be found, until we reach comets and meteorites where it is found in huge quantities. Astrochemists measure the amount of sulfur found in regards to solar abundance, which is our best indicator of elemental distribution in our galaxy.
From observations of the interstellar medium, we can only retrieve at least 1% of the total amount expected of sulfur, whether it is taking the form of gas or solid. That means the other 99% remains unidentified or hidden in different forms. Astronomers can mainly find sulfur in a solid phase, such as ices or dust. So far, only two sulfur-bearing molecules in ices have been identified, representing about 4% of the missing sulfur. To this day, we don’t know where and in what form sulfur is hidden.
Searching for the invisible
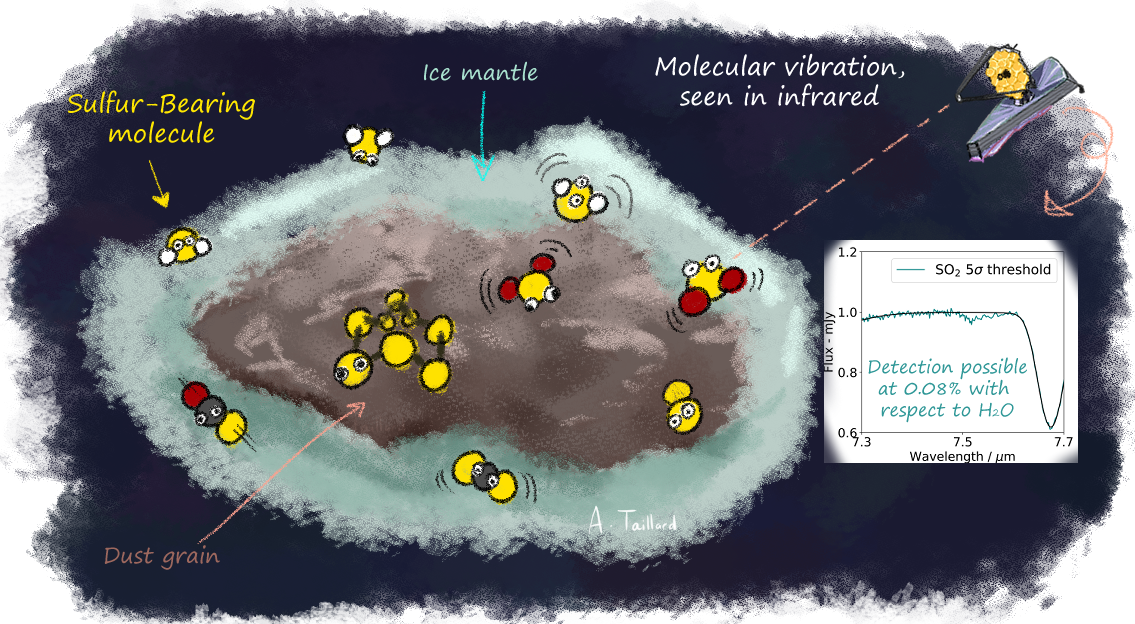
Thanks to the sensitivity of the James Webb Space Telescope (JWST), ice observation has reached a new milestone. This telescope can probe through the different phases of the interstellar medium (ISM) and can be used to find missing sulfur. On Earth, laboratory experimentalists are able to reproduce the critical physical conditions that are encountered in space (vacuum and temperatures lower than -263°C/-441.4°F). These scientists study how atoms and molecules interact and form new molecules, as would be expected in the ISM. They can help to identify what kind of environments could be observed with the JWST, from the beginning of the star formation to its later stages. Usually, astrochemists are able to predict what kind of molecule will be dominant under specific conditions. If a specific molecule is expected to be more abundant than the other, then it is called a "reservoir”. Combining laboratory experiments and astrochemical models, this team is determining the reservoir in which sulfur could be found. They have discovered that a few species, ranging from simple (H2S, SO2, CS2, and OCS) to long (S3, S4, S5, and S8) molecules could be considered reservoirs in different phases of star formation.
Figure 1 : Illustration of an ice-coated dust grain in the interstellar medium, where sulfur-bearing molecules (the sulfur atoms are yellow) vibrate at the surface of the grain. This vibration can be observed with the JWST in the near-infrared, where we show an example of a synthetic spectrum of the expected observation, with the amount of molecules needed to secure detection.
Unfortunately, considering how weak the signal of each molecule is, detection is unlikely in certain environments, meaning that finding the entirety of the missing sulfur is still far out of reach. However, a single detection of any of these molecules could lead to a giant leap in our understanding of sulfur chemistry and, most importantly, its role in the appearance of life on Earth.
This article made use of the following publication:
Taillard. A. et al., 2025, Astronomy & Astrophysics, 694, A263
Original Contributor

Angèle Taillard
Centro de Astrobiología (CAB), CSIC-INTA
Angèle is a postdoctoral researcher focusing on the surface chemistry of interstellar dust grain and improving astrochemical models. Affiliation: Centro de Astrobiología (CAB), CSIC-INTA, Ctra. de Ajalvir, km 4, Torrejón de Ardoz, 28850 Madrid, Spain